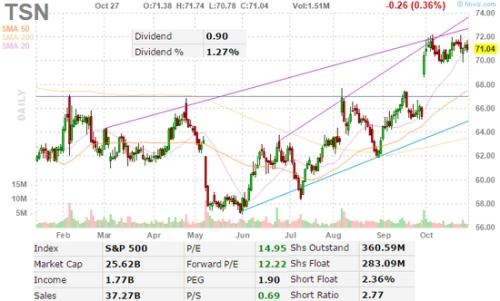
In the world of pharmaceuticals and biotechnology, the FDA approval is a monumental milestone. The U.S. Food and Drug Administration (FDA) is known for its rigorous review process, ensuring that only safe and effective drugs make it to the market. Recent OC GN news has been abuzz with FDA approval breakthroughs, each heralding a new era in healthcare. This article delves into some of these significant developments and their implications for the industry and patients alike.
Revolutionary Drug Approvals: A Closer Look
Oncology Breakthroughs:
The FDA has been making waves in the oncology sector with its approval of several innovative drugs. One such breakthrough is the approval of a new immunotherapy drug for melanoma patients. This therapy has shown promising results in clinical trials, offering hope for patients who have run out of treatment options.
Genetic Therapies:
Another significant development in OC GN news is the approval of gene therapy for a rare genetic disorder. This groundbreaking treatment has the potential to correct the underlying genetic defect, leading to a permanent cure for patients. The approval marks a major step forward in the field of genetic medicine.
The Impact of FDA Approval on the Pharmaceutical Industry
The FDA's approval of these revolutionary drugs has a profound impact on the pharmaceutical industry. It validates the hard work and dedication of researchers and pharmaceutical companies. It also opens doors for new investment and innovation in the sector.
Market Potential:
The approval of new drugs and therapies creates new markets. Companies can capitalize on these opportunities by expanding their product lines and exploring new treatment options for various diseases.
Regulatory Environment:
The FDA's approval process sets a benchmark for other regulatory agencies around the world. It helps in establishing a global standard for drug safety and efficacy.
Implications for Patients
The FDA approval of these groundbreaking drugs brings hope and relief to patients. It provides them with new treatment options that were previously unavailable. This is particularly significant for patients with rare diseases, who often face limited treatment choices.
Personalized Medicine:
The approval of genetic therapies and immunotherapies highlights the rise of personalized medicine. These treatments are tailored to individual patients based on their genetic makeup, leading to better outcomes and reduced side effects.
Improved Access to Care:
The approval of new drugs and therapies increases access to care for patients. As these treatments become more widespread, more patients will have access to the latest advancements in medicine.
Case Studies: Real-world Applications
Let's take a look at some real-world applications of these FDA-approved drugs.
Immunotherapy for Melanoma:
A melanoma patient, who had tried multiple treatment options without success, was recently given the new immunotherapy drug. The treatment has shown remarkable results, significantly improving the patient's quality of life.
Gene Therapy for a Rare Genetic Disorder:
A young boy suffering from a rare genetic disorder received gene therapy after being approved by the FDA. The therapy corrected the underlying genetic defect, leading to a near-complete recovery.
In conclusion, the OC GN news of FDA approval breakthroughs is a testament to the advancements in medicine and biotechnology. These developments have the potential to revolutionize the way we treat diseases and improve patient outcomes. As we continue to witness these breakthroughs, it's clear that the future of healthcare is bright and full of possibilities.